Chapter I: Discovery of cAMP and the phosphotransferase system
In 1965, when cAMP was first discovered in Escherichia coli, it was noticed that the inhibitory effect of glucose on cAMP synthesis was also observed with various substrates, even though glucose was more effective than the other substrates (Makman RS, 1965).
In 1975, it was established that carbon sources control the intracellular levels of cAMP by regulating its synthesis (Epstein W, 1975). It was also found that intracellular levels of cAMP were not regulated by variations in the efflux rate of cAMP. Later on, Goldenbaum PE, 1979 reported the rate of cAMP excretion was possibly regulated by both the degree of membrane energization and the number of carriers present per cells.
The effect of the carbon source on cAMP levels has since been corroborated by several laboratories worldwide. E. coli cells grown in a minimal medium supplemented with any one of a variety of carbon sources exhibit different levels of cAMP. For instance, the following carbon sources are arranged in order beginning with the one leading to the lowest up to the highest level of cAMP: (1) glucose-6-phosphate, (2) glucose, (3) mannitol, (4) gluconate, (5) fructose, (6) lactate and (7) glycerol.
Variations in the cAMP level can be monitored simply by measuring the activity of β-galactosidase, which hydrolyzes lactose to glucose and galactose. The lacZ gene, encoding β-galactosidase, is part of the lac operon which is under positive control of the CRP-cAMP complex (Varmus HE, 1970). Consequently, under specific experimental conditions, there is an almost linear relationship between intracellular cAMP concentrations and β-galactosidase activities. Incidentally, the lacZ gene, due to its well-studied regulation, has been used in numerous studies including the study of adaptive mutations in E. coli (Morreall J, 2015).
A partial explanation for cAMP variations came with the study of the phosphotransferase system (PTS), which was discovered by Saul Roseman1 and coworkers (Kundig W, 1964), and further analyzed (Meadow ND, 1990). The PTS was the object of a thorough review by Postma PW, 1993, later on updated with an emphasis on Gram-positive bacteria (Deutscher J, 2006). A review by Deutscher J, 2014 was released for the 50th anniversary of the PTS discovery, which was remarkably reported in the 1964 landmark paper (Comment). Comparative genomic analyses of the PTS were reported by screening 202 sequenced genomes (Barabote RD, 2005). By using both phylogenetic methods and analysis of genome context within 222 sequenced genomes, horizontal gene transfer was implicated in the evolution of the PTS (Comas I, 2008). The PTS has been found in archaea, for example Thermofilum pendens (Fenner MW, 2008). Novel uncharacterized PTS are discovered, for example in the pathogen Salmonella enterica (Miller KA, 2013), as well as novel substrates, for example fructoselysine (Miller KA, 2015, Comment).
1Hexosamine metabolism, sialic acids, and the phosphotransferase system: Saul Roseman's contributions to glycobiology (Kresge N, 2006); Saul Roseman: His many contributions to biochemistry over eight decades (Simoni RD, 2011)
The PTS is a bacterial membrane transport system that allows the concomitant transport and phosphorylation of carbohydrates, essentially sugars (PTS-sugars). Transport and phosphorylation occur at the expense of phosphoenolpyruvate (PEP) through phosphoryl transfer between two cytosoluble proteins, Enzyme I (EI) and HPr, and the different Enzymes II which are specific for each PTS-substrate transported. The Enzymes II are characterized by their domains (A, B, C and possibly D) present either as a single polypeptide chain or as several polypeptides. A valuable uniform nomenclature for the proteins and protein domains of the PTS was proposed (Saier MH Jr, 1992). The phosphoryl transfer involves phosphorylation of histidine and cysteine residues (Jardin C, 2008).
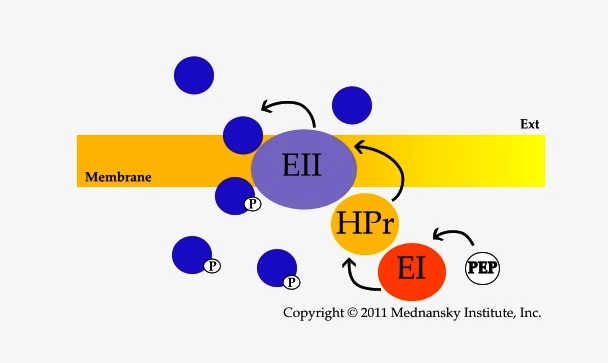
The typical PTS phosphorylation cascade from PEP to the PTS substrate, Color-coded PTS of Escherichia coli K12
Formation of stable transition state complexes between the different PTS proteins may occur during transport (Rohwer JM, 2000). The sub-cellular distribution of EI, the first phosphotransfer protein of the PTS complex, varies with growth conditions (Patel HV, 2004), an observation which later on was not corroborated (Lopian L, 2010). Kinetic studies have indicated that EI acts as a dimer and can phosphorylate HPr without dissociating to a monomer (Meadow ND, 2005). The isolated C-terminal domain of EI is able, as a dimer, to hydrolyze PEP into pyruvate and inorganic phosphate although not as efficiently as full length EI. Such domain provides a model system for studying conformational dynamics regulating EI activity (Venditti V, 2012). Effects of ligands on EI have been explained by a molecular model involving a 'swivel' upon binding of PEP (Patel HV, 2006; Teplyakov A, 2006). The interactions between Enzyme I and its inhibitor α-ketoglutarate (which accumulates during nitrogen starvation) have been studied by NMR and enzymatic assays (Venditti V, 2013). The proposal Enzyme I acts as a PEP synthase (Long CP, 2017) is questionable because it is based on unreliable data, Comment.
Binding between EI and HPr does not involve significant conformational changes, HPr acting as a phospho-relay between EI and the Enzyme II complexes (Suh JY, 2008). Motifs have been determined in HPr that are crucial, and highly specific, to the molecular interactions of HPr with its targeted Enzyme IIA domains (Reichenbach B, 2007). HPr also interacts with regulatory proteins, for example MtlR which regulates the expression of the mannitol operon (Choe M, 2017, Comment).
Additional kinetic studies of sugar binding and phosphotransfer reactions between Enzyme II domains led to model-based predictions of the kinetic behavior of the PTS, especially the glucose-PTS (Meadow ND, 2005). Enzyme II mutants have been constructed to study phosphotranfer between domains, for example mannitol Enzyme II (Opačić M, 2010). Structural studies highlight differences between the IIC domains for example IICMtl and IICChb (Opačić M, 2012) and IICGlc (Kalbermatter D, 2017).
As a related issue, it is worth mentioning the PTS has been described as a drug target system for the identification of novel and highly specific anti-microbials. Also, worth noting, a PTS permease can be necessarily required to allow for toxicity caused by secreted bactericidal compounds (Bieler S, 2006).
Considering this rather complex transport system, it can be generalized that the rate of transport of any PTS-sugar depends on the concentration of the carrier proteins and the rate of phosphate transfer between these carrier proteins. When two PTS-sugars are present in the culture medium, competition for uptake is likely to occur according to the same factors. Interestingly however, in E. coli, the PTS evolved for glucose to be taken up preferentially and the key factor in this preferential uptake is the glucose-specific IIA protein, Enzyme IIAGlc, the product of the crr (carbohydrate repression resistant) gene; Enzyme IIAGlc at UniProtKB.
Enzyme IIAGlc has been characterized by kinetic studies of different mutants including truncated forms (Meadow ND, 2006). Such studies supported the proposal that the N-terminal 18 residue domain attaches to the membrane thereby stabilizing the interaction between Enzyme IIAGlc and the IIB domain of Enzyme IICBGlc (the glucose permease) during glucose transport (Wang G, 2000), RCSB PDB for structural data (you may enter IIAGLC as keyword).
Discovery of PTS secondary regulatory functions involving Enzyme IIAGlc, i.e., regulation of adenylate cyclase and the phenomenon of inducer exclusion (Saier MH Jr, 1975), provided an explanation for the preferential uptake of glucose over other sugars, particularly non-PTS-sugars, by E. coli. It also provided an explanation for the exemplary glucose-lactose diauxie ('croissance double' as coined by Monod J, 1942, two distinct exponential phases separated by a complete cessation of growth), even though a role for cAMP in diauxie had been rejected by Kimata K, 1997 (Crasnier-Mednansky, 2008). Is cAMP a key element in diauxie? (Commentary 1). Unfortunately, the role of cAMP in diauxie is still not considered (Chu D, 2016, Comment), and diauxie is misunderstood (Kotte O, 2010, Comment; Ni L, 2013, Comment; Enjalbert B, 2015, Comment). The role of cAMP is also ignored for other diauxic growth, for example the glucose-acetate diauxie (Spencer CC, 2007, Comment), and data indicating addition of cAMP does not relieve glucose repression should be analyzed with caution (Baptist G, 2013, Comment). Monod's characterization of diauxie remains exemplary, and establishes specific growth parameters (Comment for Bren A, 2016).