The MalX protein of Escherichia coli
The Escherichia coli protein designated MalX is homologous to Enzyme IICBGlc, the two-domain glucose permease of the glucose phosphotransferase system or glucose PTS (Reidl J, 1991), MalX at UniProtKB.
The CRP-cAMP complex, as a Class II activator, activates the transcription of malX encoding MalX (Hollands K, 2007; Lloyd GS, 2008).
Over-expression of malX was shown to allow transport of glucose (and maltose) with concomitant phosphorylation in strains lacking Enzyme IICBGlc and Enzyme IICMan and IIDMan (which are mannose-specific but can be used for glucose transport). It was then proposed that MalX could be phosphorylated by Enzyme IIAGlc (Reidl J, 1991).
When malX is expressed constitutively, growth on glucose is dependent on glucokinase. Therefore MalX can also mediate glucose transport without concomitant phosphorylation.
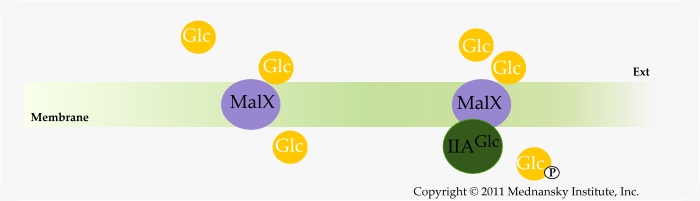
The lost routes for glucose utilization: MalX-facilitated diffusion and MalX-dependent PTS-type of transport
It can be reasoned that, if over-produced MalX is phosphorylated by Enzyme IIAGlc, adenylate cyclase should be inactive during glucose transport by MalX. Indeed, mutant strains lacking Enzyme IICBGlc, Enzyme IICMan and IIDMan, and glucokinase, and over-producing MalX, show a low level of cAMP when grown on glucose (as compared to other carbon sources). Furthermore, in such strains, Enzyme IIAGlc is required for MalX-mediated transport of glucose (Crasnier-Mednansky M, unpublished data).
The interesting feature of MalX is its capability to transport glucose with or without concomitant phosphorylation, however with much less efficiency than Enzyme IICBGlc. Such feature seems to weaken the contention that PTS permeases cannot transport their substrates without concomitant phosphorylation. Within the same frame of thought, it was reported fructose could be transported by facilitated diffusion via the glucose PTS however only in certain E. coli mutant strains (Kornberg HL, 2000).
In uropathogenic E. coli (UPEC), MalX was implicated in pathogenesis, particularly biofilm formation, and thus was designated PafP (Patho-film regulator P). In particular, deletion of malX (pafP) prevented urinary tract infection in mice (Baum M, 2014).